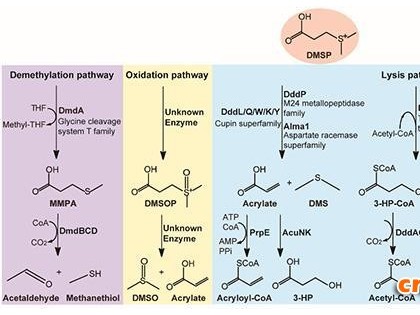
AlkB型蛋白是脱甲基酶,被认为通过氧化性去除DNA、RNA和组蛋白上的甲基加合物而在DNA修复中扮演一个角色。Yi等人确定了在与各种不同的被修饰的DNA形成的复合物中结晶了的AlkB氧化酶的结构。
通过在厌氧条件下生长晶体,然后将它们暴露于双氧以启动氧化,他们捕捉到两种不同的中间体。第三种类型的中间体是另外利用计算分析确定的。这些结构为这些酶怎样进行氧化脱甲基反应提供了详细的机制性见解。(生物谷Bioon.com)
生物谷推荐英文摘要:
Nature doi:10.1038/nature09497
Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase
Chengqi Yi,Guifang Jia,Guanhua Hou,Qing Dai,Wen Zhang,Guanqun Zheng,Xing Jian,Cai-Guang Yang,Qiang Cui& Chuan He
Mononuclear iron-containing oxygenases conduct a diverse variety of oxidation functions in biology1, 2, including the oxidative demethylation of methylated nucleic acids and histones3, 4. Escherichia coli AlkB is the first such enzyme that was discovered to repair methylated nucleic acids5, 6, which are otherwise cytotoxic and/or mutagenic. AlkB human homologues are known to play pivotal roles in various processes7, 8, 9, 10, 11. Here we present structural characterization of oxidation intermediates for these demethylases. Using a chemical cross-linking strategy12, 13, complexes of AlkB–double stranded DNA (dsDNA) containing 1,N6-etheno adenine (εA), N3-methyl thymine (3-meT) and N3-methyl cytosine (3-meC) are stabilized and crystallized, respectively. Exposing these crystals, grown under anaerobic conditions containing iron(II) and α-ketoglutarate (αKG), to dioxygen initiates oxidation in crystallo. Glycol (from εA) and hemiaminal (from 3-meT) intermediates are captured; a zwitterionic intermediate (from 3-meC) is also proposed, based on crystallographic observations and computational analysis. The observation of these unprecedented intermediates provides direct support for the oxidative demethylation mechanism for these demethylases. This study also depicts a general mechanistic view of how a methyl group is oxidatively removed from different biological substrates.